The arrival and rollout of the Oxford-AstraZeneca vaccine, the first COVID–19 vaccine procured by Sri Lanka, is a small but optimistic step towards protecting the local population against the disease, and minimising the spread of the virus. As priority groups begin receiving their first doses of the vaccine, however, members of the public have posed important questions regarding its safety, efficacy, side effects, and more. Below are the answers to some of the most commonly asked questions about COVID–19 vaccines, based on a report published by the World Health Organization (WHO), Sri Lanka.
Safety And Immunity
Are COVID–19 vaccines safe?

Yes. While COVID–19 vaccines are being developed quickly, safety remains one of WHO’s highest priorities. Standard safety and quality monitoring mechanisms are strictly adhered to, and the WHO works closely with national regulatory authorities to ensure that the vaccines comply with global norms and standards.
Do COVID-19 vaccines provide long–term immunity?

Since the vaccines are new, it remains unsure as to whether they will provide long–term immunity against COVID–19.
How effective are COVID–19 vaccines?
While many COVID–19 vaccines have high levels of efficacy, none are 100% protective against the virus. Therefore, a small percentage of people, due to their genetic or biological characteristics, will not develop immunity as expected after receiving the COVID–19 vaccination.
Additionally, several other factors — including a person’s age, underlying health conditions, or previous exposure to COVID–19 infection — may affect the extent to which the vaccine will protect them from COVID–19.
Can a person still contract COVID–19 after receiving the vaccine?

Following the vaccination, it can typically take up to a few weeks for the body to develop immunity against the virus. This means it is possible for a person to become infected with the virus and fall sick immediately before or after receiving the vaccine. This is because the vaccine has not had enough time to provide protection, not because it is ineffective.
Can a person get COVID–19 through vaccination?

No. None of the authorised COVID–19 vaccines nor any that are currently in development contain the live virus that causes COVID–19. Therefore, none of the COVID–19 vaccines can cause the disease.
If a person already had COVID–19 and recovered, do they still need to get vaccinated?

Yes. Reinfection with COVID–19 is a possibility, and due to the severe health risks associated with the disease, vaccines should be offered to all people regardless of whether they have or have not previously contracted COVID–19.
Natural immunity — the immunity someone gains from having an infection — varies from person to person. Both natural immunity and vaccine–induced immunity to COVID–19 are important aspects of the virus that experts are trying to learn more about. At present, whether you have contracted COVID–19 or not, precautions against infection should be taken by wearing a mask, maintaining social distancing, washing your hands frequently, and avoiding crowds and confined spaces.
Vaccines And Regulation
How are COVID–19 vaccines regulated and approved for use?
Vaccines are only introduced into national healthcare systems following regulatory approval and thorough quality control. After a vaccine has been developed, manufactured, and undergone clinical testing to ensure safety and efficacy, manufacturers must submit data to be assessed by regulatory agencies to authorise use of the vaccine.
Manufacturers can submit their products to a national regulatory authority, a stringent regulatory authority (SRA), or to the WHO for prequalification or WHO emergency listing (EUL). An SRA is a national drug regulatory authority that is considered by WHO to implement stringent standards for quality, safety and efficacy in its review of drugs or vaccines for marketing authorisation.
Will Sri Lanka receive a single type of vaccine?
Sri Lanka is a member of the COVAX Facility, which is the vaccine pillar of The Access to COVID-19 Tools Accelerator (The ACT–Accelerator), the only global framework for ensuring the fair and equitable allocation of COVID–19 tools such as tests and vaccines. Since the COVAX Facility is considering multiple vaccines for distribution among participating countries, Sri Lanka may receive more than one type of vaccine.
National authorities will decide how to use a country’s allocated doses of vaccines based on the country’s COVID–19 situation, guidance from national policy–making bodies, and recommendations and advice from the WHO Strategic Advisory Group of Experts on Immunization (SAGE).
Which vaccines will we receive through COVAX?
COVAX will consider procuring any candidate vaccine that meets the global standards set by WHO. As part of the COVAX research and development portfolio, the Coalition for Epidemic Preparedness Innovations (CEPI) has invested in ten vaccine candidates, nine of which are in development and seven of which are in clinical trials. Among the candidates are Oxford–AstraZeneca, Moderna (USA), Novavax (USA), and CureVac (Germany).
Can one vaccine be interchangeably administered with another vaccine?
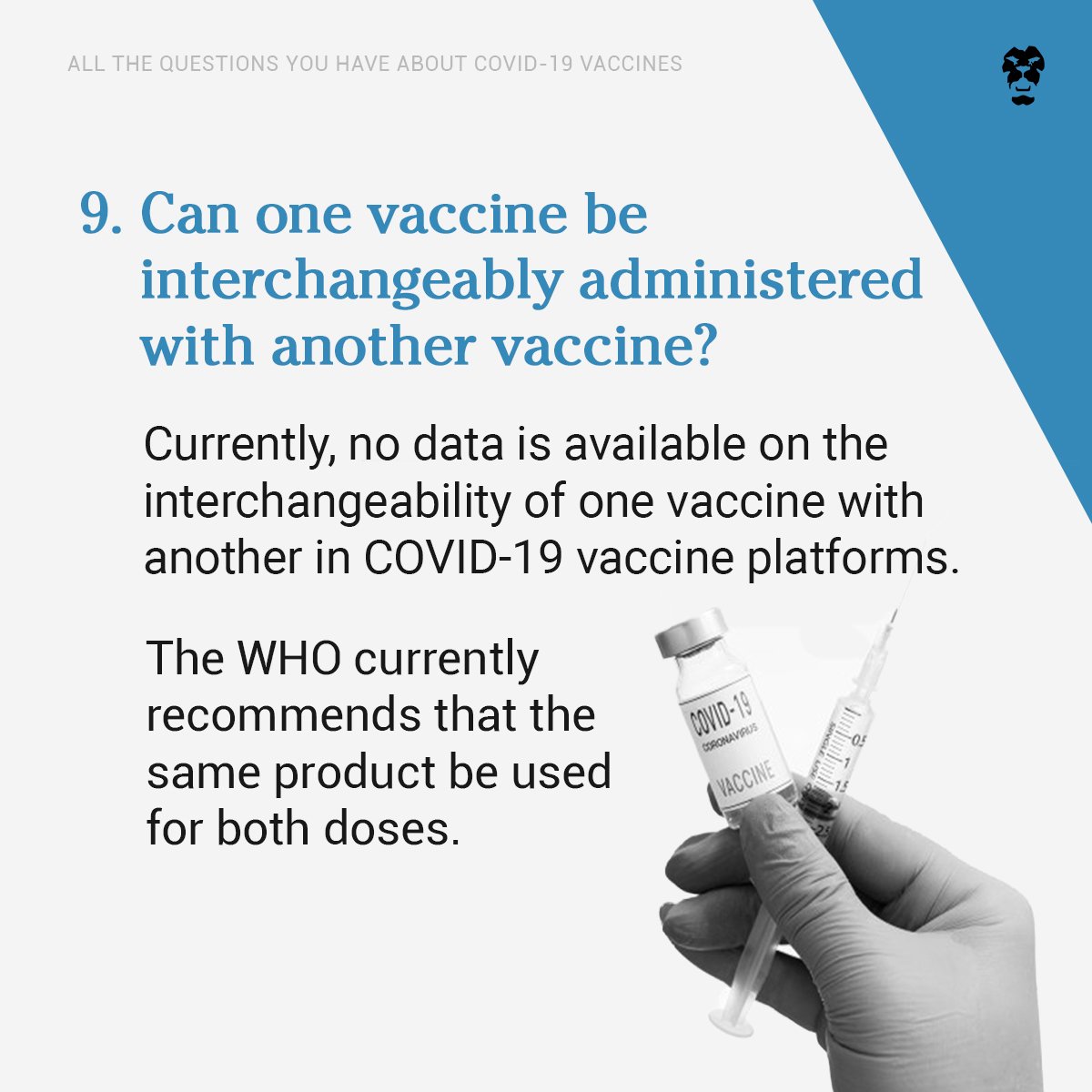
Currently, no data is available on the interchangeability of one COVID–19 vaccine with another. The WHO recommends that the same product be used for both doses.
Administration And Distribution
How are COVID–19 vaccines administered?
Vaccines are given intramuscularly via injection. Most currently available vaccines recommend two doses, with an interval of 21–28 days between doses.
Do COVID–19 vaccines have any side effects?

Like any vaccine or medicine, COVID–19 vaccines can cause mild side effects such as fever, headache, tiredness, muscle pain, and/or pain or redness at the injection site. Severe or long–lasting side effects are extremely rare, and mild reactions disappear within a few days.
Who will receive the COVID–19 vaccine first?
As the supply of vaccines will be limited during the initial phase, to ensure equitable access for vaccines, WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) have issued policy recommendations on population prioritisation.
SAGE recommends prioritising frontline workers (healthcare workers and others), older people, and those with certain pre–existing health conditions that may increase the severity of COVID–19 symptoms if they are infected with the virus. Countries may recommend more high–risk groups if they feel it is urgent to vaccinate these groups in the initial phase.
Who will not receive the COVID–19 vaccine?

Due to the lack of data on the safety of the COVID–19 vaccine in the following groups of people, the vaccines are not recommended for children and adolescents below the age of 16 years, pregnant women, and people with current acute COVID–19. However, with evolving research and evidence, these decisions may be reviewed and revised.
Does the development of COVID–19 vaccines mean that the pandemic is over?

While vaccines are important tools, they will not end the pandemic by themselves. As vaccines are distributed globally, we need to take all necessary measures to prevent the virus from spreading and causing more deaths. This means that, in addition to the vaccine rollout, we need to continue wearing masks, social distancing, washing hands regularly, avoiding crowds, disinfecting surfaces, and staying at home if asked to.
Additionally, while vaccines are effective against developing COVID–19, we don’t yet know their impact on preventing transmission of the disease. As ongoing studies help answer these questions, it is important that anyone who receives the vaccine continues to take the necessary precautions to protect everyone in the community.
.jpg?w=600)






