
Although Sri Lanka was declared malaria-free by the WHO in 2016, the country has struggled with combatting another mosquito-borne virus—dengue.
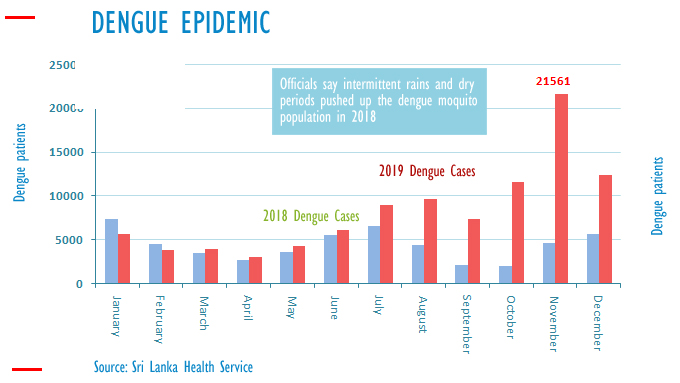
Despite various schemes being implemented over the years, in 2019 alone, 103, 924 suspected cases were reported to the Epidemiology Unit of the Ministry of Health—the highest number from the Colombo district.
Spread by females of the Aedes type of mosquito, most commonly the Aedes aegypti, symptoms of dengue, which begin four to six days after infection, include high fever, headaches, nausea, muscle and joint pains, mild bleeding, and a skin rash. While most people recover between two to seven days, for some it develops into more severe forms known as dengue hemorrhagic fever, or dengue shock syndrome, which involves heavy bleeding and dangerously low blood pressure.
Vaccines for dengue exist, but it is only recommended to those who have been previously infected, and prevention methods are usually more focused on reducing the Aedes aegypti mosquito which spreads the disease.

In Sri Lanka, the government has for many years conducted education and awareness programmes to limit the spread of dengue, but it has been an uphill task.
Four new developments may revolutionise the fight against the disease. But first a little about bioinsecticides.
Bioinsecticides
The use of chemical insecticides is a common way to kill mosquitos and combat the spread of disease. However, they are often only short-term solutions, and can only be used sparingly without the risk of damaging other life forms.
Bioinsecticides are a combination of biological controls and insecticides. One example of a bioinsecticide is Bacillus thuringiensis israelensis (Bti), which is a naturally occurring soil bacterium that can effectively kill mosquito larvae present in water.
There are many strains of Bacillus thuringiensis, each with unique toxicity characteristics, and Bti is very specifically used against mosquitoes. It is available in small, slow-release bricks called ‘mosquito dunks’ that float on the water surface and are effective in treating deep water. Other bioinsecticides, such as pyriproxyfen and methoprene, act as juvenile hormone analogues that prevent mosquito larvae from metamorphosing into adults.
Recently, researchers used mosquitoes to transfer insecticides to larval habitats. They noticed that after a blood meal, female Aedes aegypti enjoy resting in damp and dark areas. To take advantage of this behavior, the researchers set up dark, damp stations dusted with a bioinsecticide that targets larvae. When the mosquitoes came to rest on the stations, their legs picked up the bioinsecticide and transferred it to the aquatic mosquito habitats where they laid their eggs. This method is effective in killing the mosquito larvae and reducing the number of adult mosquitoes.
Sri Lanka first used Bti in 2010, following the recommendation of Cuban experts to control dengue in the country. The company Labiofam Pharmaceutical Biological Laboratories Enterprise of Cuba, sold 30, 000 litres of the bioinsecticide to be used around the city of Kandy.
Bti is still used around Sri Lanka, but at a small scale. A study carried out in Colombo in 2018 found the bioinsecticide did reduce the mosquito population in the area significantly.
However, it was also noted that after 8 weeks, the population had returned to normal, and that like other insecticides, the residual effects of Bti were limited. The conclusion of the study was that Bti would have to only “play a supplementary role to other vector control methods.”

Drones and Machine Learning
Which is why, in conjunction with drones and machine learning technology, bioinsecticides like Bti could be made to be much more effective.
A recent research project successfully used drones to identify breeding sites of mosquitoes. Drones are cost-effective and able to access remote and difficult to reach places, such as roof gutters, overhead water tanks and inaccessible rooftops.
Potential water retention areas, which can be identified using indicators like lichens which thrive in stagnant water and are visible because of their dark colour, can then be labelled and scouted.
Locating such mosquito breeding sites is useful for the Public Health Inspectors to release Bti bacteria with spores that produce toxins aimed at attacking mosquito larvae.

The Wolbachia Bacteria
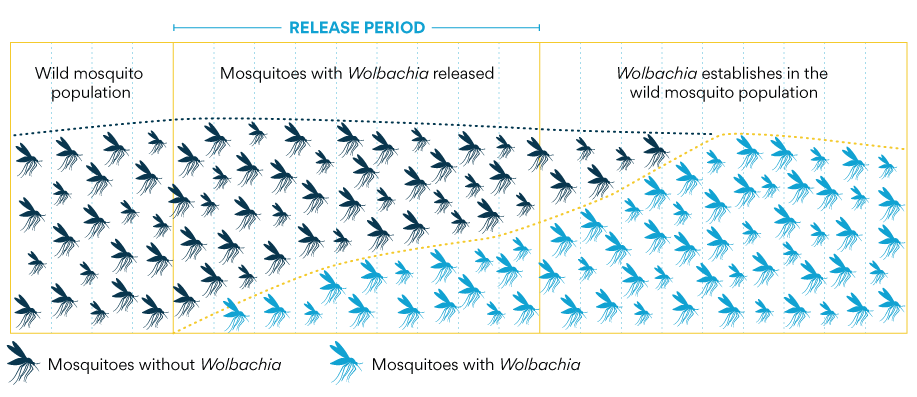
The World Mosquito Program in Sri Lanka has begun pilot projects to use another pathogen, the Wolbachia Bacteria, to inhibit the spread of dengue among mosquitos.
The method was first implemented in Australia, where no transmissions have been recorded in eight years, and has since been used successfully in 12 countries including India.
The initiative in Sri Lanka is supported by the Australian government, and if successful, could serve as a model for future large-scale implementation of a low-cost and self-sustaining method for the prevention of mosquito-borne diseases.
The method works by infecting a sample of Aedes aegypti mosquitoes with the Wolbachia bacteria. When Aedes aegypti mosquitoes carry Wolbachia, the bacteria compete with other viruses like dengue, Zika, chikungunya and yellow fever. This makes it harder for viruses to reproduce inside the mosquitoes, and the mosquitoes are much less likely to spread viruses from person to person.
The Wolbachia-infected mosquitoes are then released into the wild where they spread the disease among other mosquitos, protecting them from infection from dengue. Risk assessments have found that Wolbachia is safe for people, animals and the environment.
Deputy Director-General of the Health Ministry, Paba Palihawadana, speaking to The News Minute last December, said that Wolbachia infected mosquitoes would be released in 25 local townships in Colombo, starting February 2020. Households would be provided with mosquito eggs and a food capsule which must be sealed in water for two weeks, after which the mature mosquitoes will be released into the environment to spread the bacteria.
Genetic Modification
Further research has investigated the possibility of genetically modifying mosquitoes to make them less likely to spread the disease, or even unable to breed.
Molecular biologist Omar Akbari of the University of California, San Diego and his colleagues re-engineered the human anti-dengue antibody to simplify its structure, making its gene easier to insert into the mosquito genome.
They then injected the simplified antibody gene into the embryos of Aedes aegypti mosquitoes, which spread dengue, and bred the resulting insects to make offspring with two copies of the new gene, which is activated only when blood enters the gut. When the engineered mosquitoes drank blood infected with any one of the four dengue serotypes, they had no detectable dengue virus in their saliva.
In the lab, these genetically-engineered mosquitoes are able to mate and produce healthy offspring. They developed slightly slower than typical mosquitoes, and the females have slightly shorter life spans. However, it’s still too early to gauge from these initial tests how fit these mosquitoes will be compared with their wild counterparts.
Further attempts have been made to genetically modify mosquitoes in order to make them infertile. The female mosquito is given a gene that either passes on to its offspring or makes it sterile, with the intention of slowly reducing mosquito populations.
A sample of the mosquito breed has been brought to Sri Lanka ‘on an experimental basis’, and the testing is currently underway. However, since this method is still in development, it is likely to face many obstacles.







